Sci Transl Med:“液体活检”可预测前列腺癌患者是否对治疗有抵抗性
 2016-05-25来源:未知
2016-05-25来源:未知伦敦癌症研究中心研究人员用一种被称作“液体活检”的方法在一个前列腺癌患者亚组中发现了可能引起耐药性的基因突变;所谓“液体活检”指的是对血液中的痕量肿瘤DNA进行检视。这些发现提示,可对患者的这些基因改变进行测试以期在治疗开始前预测肿瘤是否会有耐药性,这可以让医生为患者量身打造最有效的治疗策略。罹患晚期前列腺癌的病人常常接受肿瘤雄激素剥夺疗法,雄激素已知会刺激前列腺肿瘤生长。大多数患者最终会产生耐药性,他们接着会接受阻断雄激素受体信号转导的新型药物。然而,这些药物只会有大约一年左右的有效时间,届时耐药性会再度出现。肿瘤组织的基因组测序可发现产生耐药性的基础基因突变,它具有指导靶向疗法的潜力,但它需要病人接受多次、侵入性的活检。lessandro Romanel和同事在此用液体活检来打捞肿瘤脱落到血液中的DNA片段,它们被称作循环肿瘤DNA。他们对97名有耐药性的前列腺癌患者的274个血液样本进行了的基因组测序,这些样本是在用一种以雄激素受体为标靶的药物治疗之前和治疗期间采集的。他们发现,即使在治疗开始之前,许多最终产生耐药性的病人体内有反常的雄激素受体基因,尤其是他们有基因拷贝数改变和体细胞突变等异常。与具有正常雄激素受体基因的患者相比,这些病人更有可能对治疗反应不佳并有更差的存活率。一篇由Michael Schweizer和Emmanuel Antonarakis撰写的相关《焦点》更详细地讨论了这些发现,其中包括用液体活检来指导对癌症病患的更精确的诊断和治疗的希望和挑战。
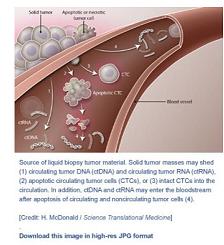
说明: 液体活检肿瘤材料来源。实体肿瘤块会有(1)循环肿瘤DNA(ctDNA)和循环肿瘤RNA(ctRNA),(2)凋亡循环肿瘤细胞(CTCs),或(3)完整CTCs脱落至血液循环之中。此外,ctDNA和ctRNA也会在循环肿瘤细胞和非循环肿瘤细胞凋亡后进入血流(4)
原文链接:Plasma AR and abiraterone-resistant prostate cancer
原文摘要:Androgen receptor (AR) gene aberrations are rare in prostate cancer before primary hormone treatment but emerge with castration resistance. To determine AR gene status using a minimally invasive assay that could have broad clinical utility, we developed a targeted next-generation sequencing approach amenable to plasma DNA, covering all AR coding bases and genomic regions that are highly informative in prostate cancer. We sequenced 274 plasma samples from 97 castration-resistant prostate cancer patients treated with abiraterone at two institutions. We controlled for normal DNA in patients’ circulation and detected a sufficiently high tumor DNA fraction to quantify AR copy number state in 217 samples (80 patients). Detection of AR copy number gain and point mutations in plasma were inversely correlated, supported further by the enrichment of nonsynonymous versus synonymous mutations in ARcopy number normal as opposed to AR gain samples. wheras AR copy number was unchanged from before treatment to progression and no mutant AR alleles showed signal for acquired gain, we observed emergence of T878A or L702H AR amino acid changes in 13% of tumors at progression on abiraterone. Patients with AR gain or T878A or L702H before abiraterone (45%) were 4.9 and 7.8 times less likely to have a ≥50 or ≥90% decline in prostate-specific antigen (PSA), respectively, and had a significantly worse overall [hazard ratio (HR), 7.33; 95% confidence interval (CI), 3.51 to 15.34; P = 1.3 × 10−9) and progression-free (HR, 3.73; 95% CI, 2.17 to 6.41; P = 5.6 × 10−7) survival. evaluation of plasma AR by next-generation sequencing could identify cancers with primary resistance to abiraterone.



